Optoribogenetics
The usage of light in the context of the cellular environment enables a reversible non-invasive trigger, that allows for precise manipulation of molecular biological functions. The photoreceptor PAL reversibly changes its conformation in the presence of blue light and switches back to its dark conformation in the absence of blue light. It binds sequence-specifically short RNA aptamers with around 20 nM affinity in blue light and weaker than 1 µM in darkness [1]. This interaction allows for a direct and light-dependent interaction between proteins and RNAs. We incorporated the aptamer in the 5’ UTR of a reporter gene mRNA. When PAL was co-expressed, the translation of the gene was attenuated under blue light. We also replaced the loop-region of micro RNAs (miRNA) and short hairpin RNAs (shRNAs) with the aptamer sequence, which resulted in a decreased DICER cleavage rate and increased reporter gene expression in the presence of blue light [2]. In conjunction with CRISPR/dCas9, the aptamer was inserted into the stem-loops of single guide RNAs (sgRNA). PAL was fused to the transcriptional activators P65 and Heat Shock Factor 1 (HSF1). When the dCas9/sgRNA complex was programmed to bind upstream of a reporter gene promoter, the expression of the reporter was upregulated when blue light was applied [3].
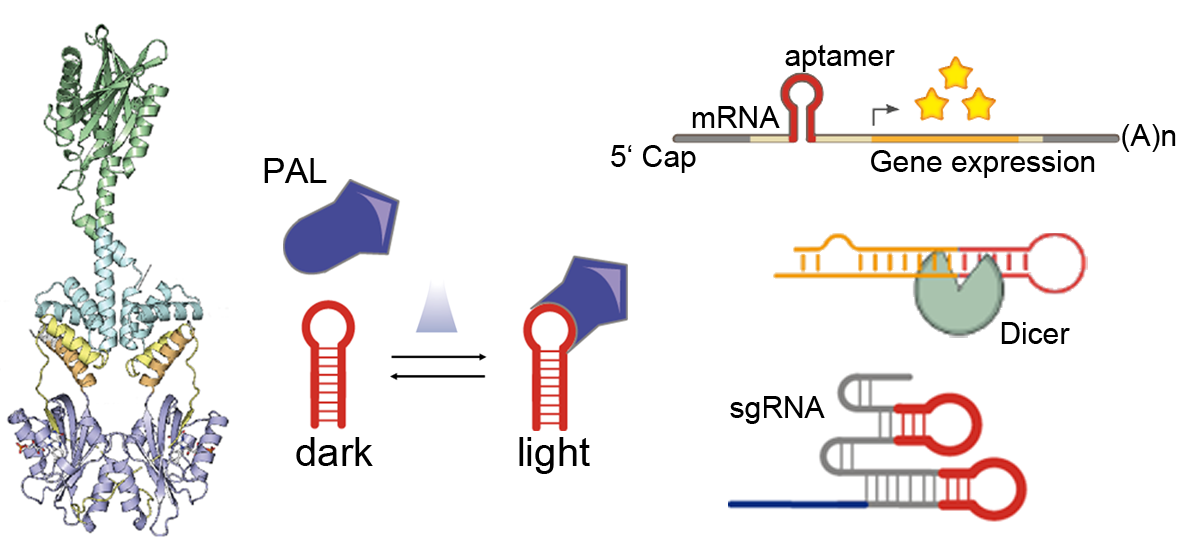
Figure 1: The photoreceptor PAL binds to a small hairpin-aptamer under blue light with high affinity, whereas in darkness binding is greatly reduced. The light-dependent interaction was used in the context of mRNA translation, DICER cleavage and CRISPR/dCas9 gene activation.
- Weber, A.M., et al., A blue light receptor that mediates RNA binding and translational regulation. Nat Chem Biol, 2019. 15(11): p. 1085-1092.
- Pilsl, S., et al., Optoribogenetic control of regulatory RNA molecules. Nat Commun, 2020. 11(1): p. 4825.
- Renzl, C., A. Kakoti, and G. Mayer, Aptamer-Mediated Reversible Transactivation of Gene Expression by Light. Angew Chem Int Ed Engl, 2020. 59(50): p. 22414-22418.




