Research
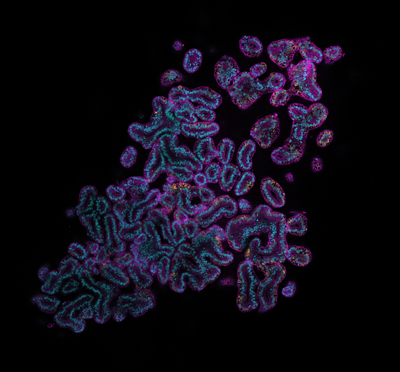
In our previous published work, we developed a robust and reliable protocol for the generation of murine bronchioalveolar lung organoids (BALO). This model was generated by FACS cell sorting and co-cultivation of bronchoalveolar epithelial stem cells and resident mesenchymal cells isolated from the lung homogenate of adult mice. After 21 days of culture, BALO spatially organized in bronchiolar and alveolar regions with airway-like structures containing basal cells, secretory, and ciliated cells while alveolar-like regions differentiate into mature alveolar epithelial cell type 1 and type 2.
The BALO model is well-suited to support infection and replication of selected respiratory viruses such as influenza (IAV). Microinjection of IAV into BALO airway-like structures recapitulates proximal-to-distal spread of the infection and causes substantial loss of AEC in the infected alveolar-like areas, thus, mimicking this aspect of IAV pneumonia. Notably, we have successfully standardized a microinjection technique that permits the direct addition of cells such as alveolar macrophages and monocytes within the lung organoids allowing investigation of cell-specific crosstalk/interactions occurring during viral injury and repair. Direct infection of BALO epithelium with different strains of IAV not only supports viral spread and replication but also elicits an enhanced antiviral immune response when micro-injected alveolar macrophages are present.
Based on these findings, our lab will employ this and other primary lung organoid systems to identify the microenvironmental factors that determine immune cells phenotype during alveolar niche development and replenishment after injury. In addition, a major aim of our lab is the establishment of human iPSC-derived lung organoids that more closely resemble the lung architecture. These complex models will be used for murine data confirmation, as well as tools for disease modeling and in regenerative medicine studies.