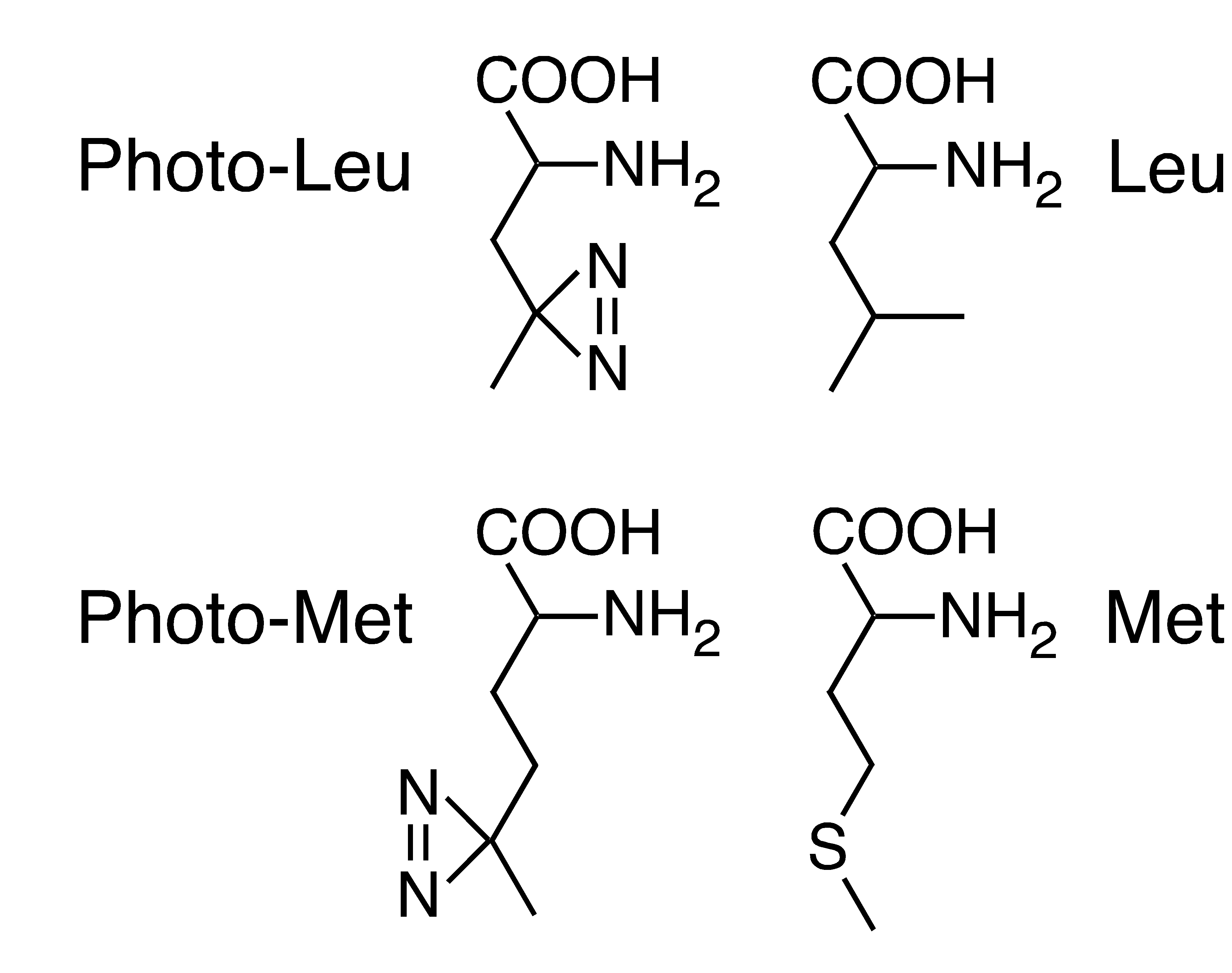
Photoactivatable Amino acids
Protein-protein interactions are the key to organizing cellular processes in space and time. The only direct way to identify such interactions in their cellular environment is photocrosslinking. We recently developed a new strategy for photo-crosslinking in living cells. Two new photoactivatable amino acids, photo-methionine and photo-leucine, that closely resemble the natural amino acids methionine and leucine, were synthesized (Suchanek et al. 2005)
These amino acids escape the stringent identity control mechanisms in protein synthesis and are incorporated into proteins by the unmodified mammalian translation machinery. Activation by ultraviolet light leads to formation of a reactive carbene that induces covalent crosslinking of interacting proteins. Highly specific detection of crosslinked proteins can be done by simple western blotting. Applying this technology to membrane protein complexes, we discovered a previously unknown direct interaction of the progesterone binding membrane protein PGRMC1 with Insig-1, a key regulator of cholesterol homeostasis.
Photo-Leu and Photo-Met are currently commercially available. Click here