Fliegen-Projekte
Cross-regulation of metabolism, innate immunity and the gut microbiome

Metazoans are in continuous contact with microorganisms, which have co-evolved with plants and animals. The microorganisms benefit the host by: determining the range of diet supply, shaping its immune system, and restricting the uncontrolled proliferation of pathogenic bacteria in the symbiotic microbial community. Interactions of the host with its microbiota occur primarily at epithelial/mucosal surfaces, such as in the gut or skin. These tissues generate barriers that prevent the invasion of pathogens into the body cavity, and also provide beneficial physiological conditions that support commensal growth. Immune responses are tightly linked to energy homeostasis and it is well established that metabolic dysregulation (e.g. due to overeating or genetic predisposition) can cause inflammation in fat and other metabolic tissues. However, exactly how they are interconnected and cross-regulated in health and disease is only just beginning to emerge. Thus, an important research line in our lab is to use Drosophila as a model organism to investigate how metabolism, innate immunity and the microbiome are cross-regulated in gastrointestinal physiology.
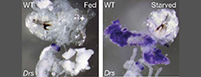
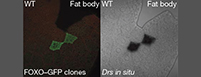
Towards this goal, we have recently discovered a novel link between metabolic and stress signaling, and innate immune effector pathways (Becker et al., Nature 2010). We found that fasting or starvation induces the expression of antimicrobial peptides in epithelial tissues, such as the gut, and that this innate immune response is observed under non-infectious conditions independent of pattern recognition receptors. In fact, starvation - which is sensed through the absence of insulin - triggers the activation of the transcription factor FOXO, which in turn, initiates antimicrobial immune responses. These findings establish an entirely novel perspective in immune sensing by directly linking the metabolic or stress status of tissues with innate effector functions of the immune system. Of note, this newly identified connection goes beyond a modulatory role of metabolic or stress signaling for immune functions, as metabolic or stress signaling pathways had not previously been implicated as initiators of immune effector functions per se. Understanding the molecular mechanisms of phenomena, such as the relationship of fasting to reduced inflammation, or the loss of appetite associated with infection, are future and timely challenges. We are currently studying the cross-regulation of metabolism and innate immunity in several Drosophila projects, including a putative significance of the FOXO-dependent mechanism for lifespan regulation.
Selected recent publication:
Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, Carrera P, Inhester T, Schultze JL, Hoch M. 2010. FOXO-dependent regulation of innate immune homeostasis. Nature 463: 369-73








